The pathological changes associated with Alzheimer’s disease begin as early as 10 to 20 years before the onset of clinical symptoms. The ability to detect these changes early in the disease continuum is crucial not only for early diagnosis and early treatment of patients but also for monitoring therapeutic response and disease progression in clinical trials.
Obtaining an accurate Alzheimer’s disease diagnosis is necessary for meeting key inclusion criteria. Currently, this is accomplished via molecular biomarkers in cerebrospinal fluid (CSF) and/or positron emission tomography (PET) scans. While highly accurate, the high cost, invasive nature, and low availability of these diagnostic procedures hamper their feasibility.
In this article, we will discuss the emergence of blood biomarkers for Alzheimer's disease and neurodegeneration and their future role in research and clinical trials.
Alzheimer’s and other neurodegenerative diseases are notoriously difficult to diagnose clinically. As such, biomarkers of the underlying pathophysiology play a crucial role in research, clinical trials, and the clinical work-up of patients.
For many years, blood biomarkers for Alzheimer’s disease were seemingly unattainable. However, thanks to great strides toward ultra-sensitive assays, blood-based tests are becoming a reality.
Alzheimer's disease is most commonly characterized by two defining features: beta-amyloid plaques and neurofibrillary tangles resulting from abnormal tau hyperphosphorylation.
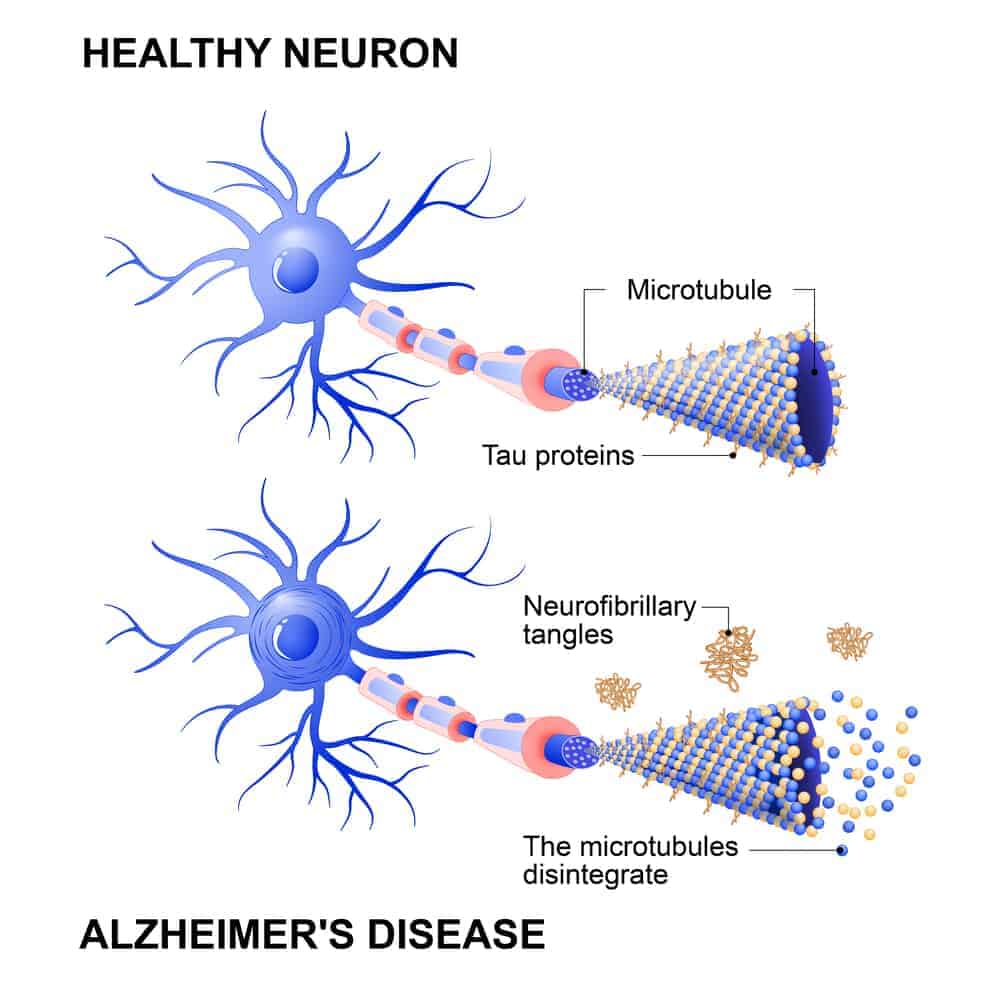
Emerging research suggests that concentrations of amyloid and phosphorylated tau proteins are associated with the corresponding concentrations of CSF as well as amyloid-PET and tau-PET scans. In particular, potential blood biomarkers for Alzheimer's disease include:
In addition to blood biomarkers for Alzheimer’s disease, researchers have also uncovered potential blood biomarkers for neurodegeneration that may provide additional information on disease progression and therapeutic response. These include:
Clinical trials are fundamental to our ability to advance Alzheimer's disease research, assess the safety and efficacy of promising new strategies for Alzheimer's disease treatment, and bring effective therapies to market. As the number of individuals living with Alzheimer's disease continues to grow at a rapid pace, Alzheimer’s disease clinical trials are more important than ever.
While the potential of emerging drugs and therapies for Alzheimer’s disease progresses rapidly, diagnostics tools and methods for robust monitoring of neurocognitive function for clinical trials remain rather outdated, driving subject recruitment costs through the roof, prolonging trial timelines, and contributing to clinical trial failures.
New, novel diagnostic and monitoring approaches, including blood biomarkers for Alzheimer’s disease, will likely be an integral aspect needed to increase the cost efficiency, speed, and success of Alzheimer’s disease clinical trials. While blood-based biomarker tests for Alzheimer’s disease are currently very expensive and difficult to access, they may in the future:
Altoida’s mission is to accelerate and improve drug development, neurological disease research, and patient care. To learn more about our precision-neurology platform and app-based medical device, contact us!